[ad_1]
Health officials have urgently recalled over 11,000 eye drops due to manufacturing issues that may have made the products unsterile that could leave patients blind.
UK medicines watchdog the Medicines and Healthcare Products Regulatory Agency (MHRA) slapped an alert on one batch of Zaditen 0.25mg/ml eye drops, used to treat seasonal allergies such as hay fever.
It is feared the drug may have been contaminated during manufacturing, potentially causing conjunctivitis and inflammation of the cornea and eyelid.
Left untreated, these conditions can cause permanent eye damage and even blindness.
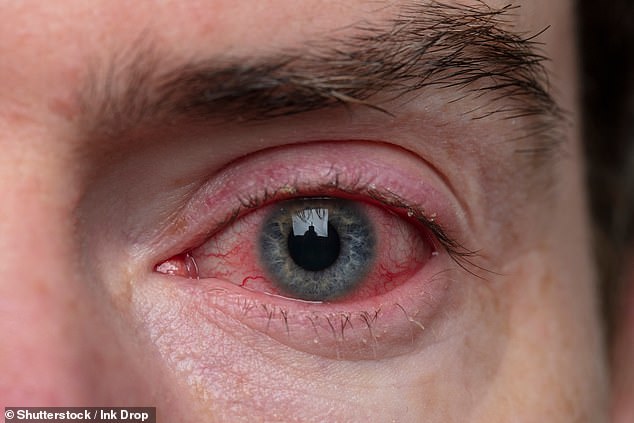
Bacterial conjunctivitis—more commonly known as pink eye—is extremely contagious.
The infection usually affects both eyes and makes them red, itchy and watery and often causes a gritty burning sensation.
In the most severe cases, this can lead to the melting and perforation of the cornea and eventually vision loss if not treated immediately with antibiotics.
But the MHRA, which published the alert today, said it had not yet received any complication or reports of harm from patients who had taken the commonly-prescribed eye drops.

The eye drops work by suppressing the immune system and dampening the allergic reaction seasonal allergy sufferers experience – but this specific batch could potentially cause inflammation and serious infection resulting in vision loss
The recall only impacts one batch of the 5ml eye drop solution, manufactured by Laboratoires Théa, with the batch number 4V64 and an expiry date of September 30 2026.
The eye drops are commonly prescribed to hay fever sufferers to help manage seasonal symptoms such as red, itchy, watery and puffy eyes.
The drops can be taken by anyone over the age of three up to two times a day.
The recall comes at a time when more people are likely to be using eye drops, as the UK experiences high pollen counts, making seasonal conjunctivitis symptoms worse.
The eye drops work by prohibiting the release of natural substances, including histamine, in the body that cause allergic reactions.
Environmental monitoring plays a critical role in developing sterile drugs, such as eye drops, allowing manufacturers to gain insight into the presence of contaminates in the environment where the drug is processed.
This helps ensure that potentially life-threatening bacteria do not come into contact with the drug.
In its recall, the MHRA said patients who experience an adverse reaction or have questions about the recall should should seek medical attention.

The recall comes amid forecasts of high pollen levels across the UK this month
Adverse side effects should also be reported via the MHRA’s Yellow Card scheme.
The scheme, set up in the 1960s, allows doctors, pharmacists and patients themselves to report adverse reactions believed to be caused by prescription and over-the-counter drugs, implants and alternative medicines.
This can lead to them being reviewed, having warnings added to the label or even being taken off the market.
Medical professionals have long urged the millions of Brits who suffer from bad hay fever and seasonal allergies to take precautions including taking regular medication.
Patients are advised to visit their local pharmacy for quick and safe treatments to relieve their symptoms—with Zaditen being a prescription only remedy.
The NHS also recommends people put Vaseline around their nostrils to trap pollen, wear wrap-around sunglasses to stop pollen getting in the eyes and shower and change clothes after being outside to wash pollen off.
Unlike a cold, which normally goes away within a week, hay fever can last for weeks or months.
Symptoms begin when immune cells mistakenly identify pollen proteins as a threat and make antibodies that trigger chemicals called histamines.
These make the blood vessels dilate, prompting the release of fluid from capillaries, triggering a runny nose, sneezing and weeping eyes.
While not life-threatening itself, tens of thousands of hay fever sufferers also have asthma, which can flare-up during pollen bombs. Asthma attacks can be fatal.
[ad_2]
This article was originally published by a www.dailymail.co.uk . Read the Original article here. .