[ad_1]
A breakthrough new drug could halt the progression of Alzheimer’s disease, claim researchers.
Trials suggest the drug—called trontinemab—could be the most powerful weapon against dementia yet, slowing down the progression of the memory-robbing disease.
Scientists will now consider whether the jab could be given to those who have not yet been diagnosed with the condition in order to prevent symptoms developing in later life.
According to research presented at the Alzheimer’s Association International Conference, Toronto, the ‘game-changing’ treatment can clear the toxic plaque thought to be behind Alzheimer’s symptoms faster than any other licensed drug.
The researchers described the findings as being ‘very promising’, adding that the drug caused far fewer side effects than existing medications.
Professor Sir John Hardy, chairman of neurological disease at University College London, told The Telegraph: ‘There is no doubt this could be game-changing.
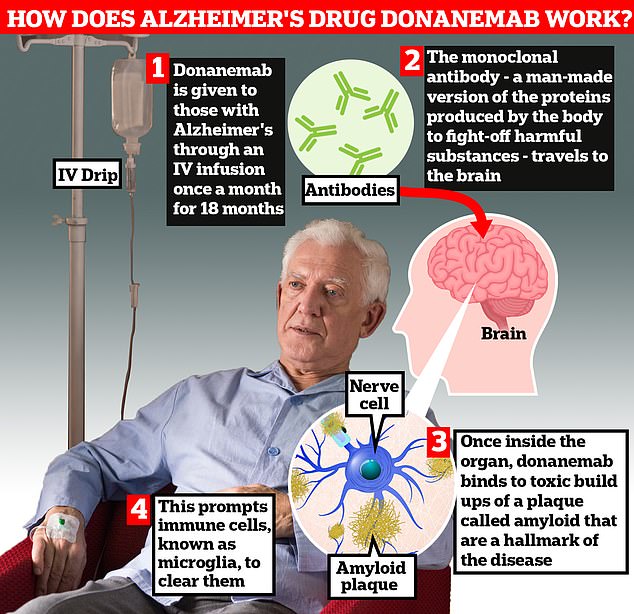
‘It sucks the plaque out of the brain really quickly, much faster then we have seen with [existing medications] lacanemab or donanemab.’
In the current trial, 90 per cent of patients prescribed the drug experienced clearance of amyloid—a toxic protein that can form plaques and tangles in the brain, interfering with memory processes—within 28 weeks of starting the treatment.
This, experts say, means visible markers of the disease had disappeared.
In a follow-up of 18-months, researchers hope these biological changes will facilitate improvements in memory and decision making, with 1,600 patients enrolled on the trial.
Currently around one million people in the UK are thought to suffer from dementia, with Alzheimer’s disease the most common form.
Recent analysis by the Alzheimer’s Society estimates the overall annual cost of the dementia to the UK is £42billion a year, with families bearing the brunt.
An ageing population means these costs, which include the lost earnings of unpaid carers, are set to soar to £90billion in the next 15 years.
But, experts hope that if given early enough, the drug could halt Alzheimer’s completely, saving some patients from developing symptoms entirely.
‘We hope if we can give these drugs to people early, we can halt the progression of the disease even before people have symptoms,’ Prof Hardy—who was the first to identify the role of amyloid in the disease—added.
And, because the drug can cross the blood-brain barrier more easily than other current treatments, promising powerful effects at low doses, it could be offered at a far lower price.

Experts have long believed donanemab could herald a new era of dementia treatment, after studies showed it slowed the memory-robbing illness in its early stages
Together with the lack of side-effects, this could see the drug become the first Alzheimer’s treatment to be funded by the NHS, experts say.
Prof Hardy continued: ‘The results show it is much fast and safer than previous drugs, which means less monitoring.
‘That brings down the cost significantly, it means fewer MRI scans, to that would surely mean it could get NICE [National Institute of Health and Care Excellence] approval.’
Last year, health watchdogs in the UK gave the green light for two so-called ‘wonder’ drugs, lacanemab and donanemab which experts claim could slow down the memory-robbing illness in its early stages.
Both the drugs use antibodies to clear toxic plaques in the brain—but some experts have warned that donanemab could cause life-threatening brain bleeds in a third of patients.
This new drug appears to be a lot safer, scientists say, with less than five per cent of patients suffering complications in the second phase of trials.
Prof Jonathan Schott, chief medical officer at Alzheimer’s Research UK said: ‘The evidence on trontinemab is very promising, showing that the drug can effectively and rapidly clear amyloid from the brain, seemingly with very few side effects.
‘We now need to see whether these early stage results carry through to later stage clinical trials, which are planned to start later this year, including in the UK.
‘These trials will show whether the drug is not only safe, but impacts on memory, thinking and quality of life.’
Levi Garraway, the chief medical officer of the manufacturing company, Roche, added: ‘With plans for phase three trials in both early symptomatic and preclinical Alzheimer’s disease, we are advancing science with the goal of delaying – and ultimately preventing – progression of this devastating condition.’
[ad_2]
This article was originally published by a www.dailymail.co.uk . Read the Original article here. .